At Jeyflex Consultants, we pride ourselves in providing world-class compliance design services to pharmaceutical companies. That’s why we were determined to achieve ISO 9001:2015 certification; the globally recognized standard for quality management (QMS). Our journey to this end was more than just a certification; it was a transformation that reinforced our commitment to delivering consistent services.
Why ISO 9001:2015?
The key principles of ISO 2001:2015 tell it all. The ISO certification journey takes you through a host of thought-provoking and mind-cracking phases of business study, in-depth comprehension of your business and operational alignment for success. Achieving this certification means following global best practices and reinforcing customers’ trust in our services.
ISO 9001:2015 Implementation Steps
Step 1: Understanding Our Baseline-Gap Analysis
To evaluate our processes, we assessed our compliance with ISO 9001:2015 and identified gaps. Fortunately, Jeyflex already has many QMSs in place, like SOPs, document protocols, and strong customer relationships. However, we found areas for improvement, including process streamlining, enhanced risk management, and greater engagement with shareholders, customers and employees.
Step 2: Employee Engagement and Training
ISO 9001:2015 places a significant emphasis on leadership and employee engagement. Consequently, we conducted frequent training sessions to guarantee that all team members were updated with quality management principles. This wasn’t just about completing tasks, it was about cultivating a culture in which everyone recognized the significance of excellence in every aspect of their work.
Our administrative and support teams, including the core processes teams, were critical to this transformation. They ensured that all documentation was up-to-date and that internal audits were conducted according to the audit programme.
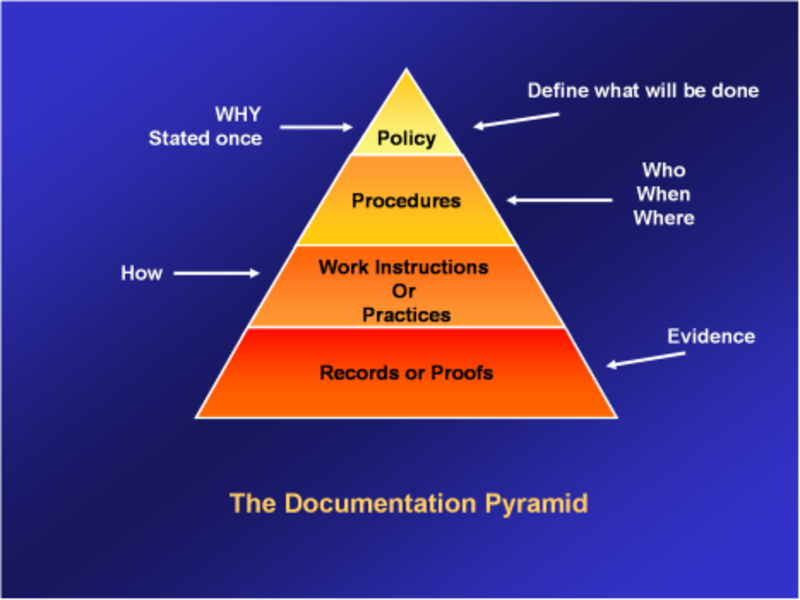
Step 3: Setting Up the QMS Framework (Documentation)
After reviewing the gap analysis results, a cross-functional team was formed to monitor compliance with the ISO 9001:2015 framework. With clear goals and timelines in mind, we began the journey of updating our processes to meet ISO requirements.
Some of the changes we made included:
1. Process Standardization
We standardized our processes and nonconformance management. As a result, every step of the process is documented in detail and regularly reviewed for accuracy and efficiency.
2. Customer feedback integration
Customer satisfaction is a cornerstone of ISO 9001:2015. We developed a system for collecting, analyzing and tracking customer feedback, enabling us to continuously improve our services.
3. Risk management
We integrated risk management into all our processes to comply with ISO risk-based thinking. This entailed identifying potential risks inherent in our context and processes that could potentially hinder the delivery of services and taking proactive steps to mitigate them.
Step 4: Implementation
Implementation was a crucial step where Jeyflex moved from planning to action. We integrated the ISO 9001:2015 framework into our daily operations. This phase involved rolling out standardized processes across all functions. This ensured that teams adhered to new procedures such as risk management, documentation, and quality controls.
Step 5: Certification Audit
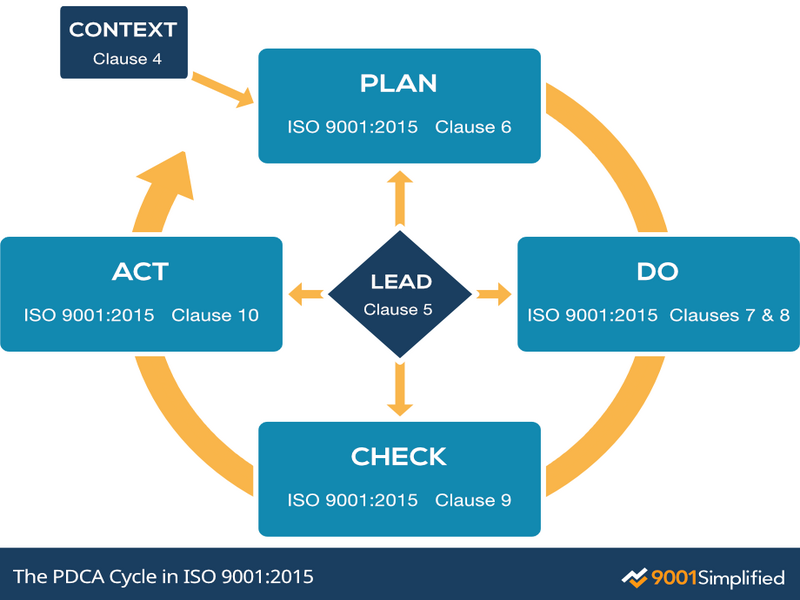
In September 2024, an external audit was conducted, the audit was comprehensive and thorough, covering every aspect of our QMS from the context of the organization, leadership, planning, support, operation, performance and evaluation and improvement. Thanks to our dedicated team and thorough preparation, we successfully passed the audit and ISO 9001:2015 certification was awarded.
Challenges Faced during the ISO 9001:2015 Certification Journey
During the ISO 9001:2015 implementation phase, we faced several challenges;
1. Balancing daily operations with ISO implementation
Concurrent establishment and standardization of internal processes and controls, while meeting customer requirements proved a hard nut to crack. Chaos was imminent.
Resolution: Phased-out implementation saved the team. We spread out the implementation workload over a longer period while delivering to clients on time every HOD took responsibility for his/her process which helped to ease the pressure.
2. Process Standardization Across Functions
Before the ISO journey, most of our functional processes were antiquated due to their origins. Building processes and procedures based on a common template was a herculean task.
Resolution: We mapped all the processes in great detail. Multi-functional teams interactively and collaboratively worked to produce standardized processes that worked. Two internal audits conducted along the journey enabled early detection of non-alignments and provided ample time for correction.
3. Maintain a harmonized documentation
One major challenge we faced was inconsistent or incomplete documentation; creating the risk of reliance on individual know-how and informal processes for service delivery.
Resolution: We developed guidelines for documentation using templates and checklists to make sure every task was comprehensively recorded and easy to review when the need arose.
4. Implementing a Risk-Based Approach
Transitioning to ISO’s risk-based thinking required a paradigm shift for most of our employees, as risk management wasn’t being consistently applied across all processes. Integrating it into daily operations was a hard tackle.
Resolution: Employees underwent training on identifying and mitigating risks, making it a core component of each department’s work. To maintain a proactive approach, regular risk assessments were introduced, ensuring that preventive measures remained a priority.
5. Audit Pressure
The certification audit put the team under a lot of pressure, as every process had to be perfectly documented. Identification of unexpected non-conformities increased the pressure, prompting the need for swift adjustments.
Resolution: We proposed to use the audit feedback as an opportunity for improvement.
Benefits of ISO 9001:2015 Certification for Jeyflex
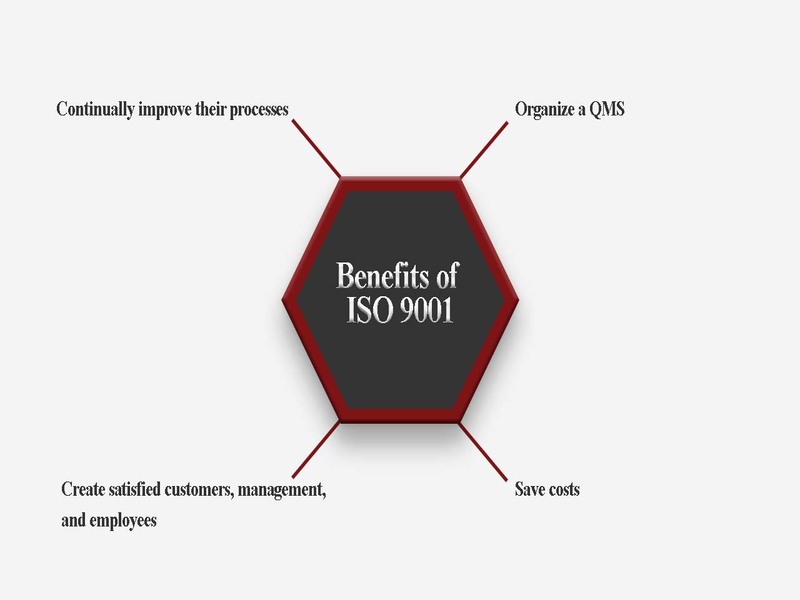
Being ISO 9001:2015 certified is more than just a badge of honour; it’s a testament to our commitment to excellence. This certification provides Jeyflex Consultants with several benefits, including;
1. Increased customer confidence – Customers now have an added layer of assurance that our processes meet international standards, reinforcing trust in our regulatory affairs, pharmacovigilance, quality assurance, training and local technical representative services.
2. Process Efficiency – Processes increase efficiency, reduce errors and streamline operations. This allows us to meet customer needs faster with reduced or no bottlenecks.
3. Continuous improvement: ISO 9001:2015 supports continuous improvement. At Jeyflex we are now committed to continuously improving our processes based on performance and customer feedback to ensure we stay ahead of the curve.
Conclusion
Achieving ISO 9001:2015 certification is an important milestone in Jeyflex Consultants’ journey, but our commitment to excellence doesn’t end here. We will continue to grow, develop and innovate to ensure our clients receive the highest level of service possible in the complex, fast-paced and ever-changing pharmaceutical landscape. This journey has not only strengthened our internal processes but also strengthened our position as a trusted partner for pharmaceutical companies seeking compliance services. Building on ISO 9001:2015 principles, we pride ourselves in driving excellence in everything we do, and we look forward to continuing to support our customers as they navigate the complex world of medicine.
Article by Marraret Ouma