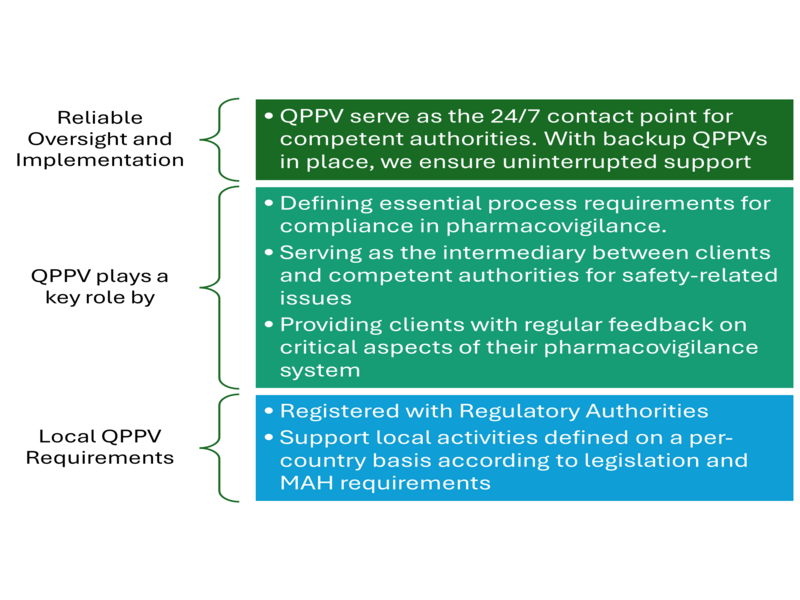
Patient safety drives the pharmaceutical industry, and the Qualified Person for Pharmacovigilance (QPPV) plays a central role in ensuring it remains a top concern by overseeing pharmacovigilance systems, monitoring product safety, and ensuring compliance with regulatory requirements
This article will explore why QPPVs are necessary to safeguard public health. Additionally, we will examine their roles and responsibilities, and discuss their importance.
Who is a Qualified Person for Pharmacovigilance (QPPV)?
A QPPV is someone with the training, knowledge and skills to ensure the safety of pharmaceutical products throughout their lifecycle. This role is legally mandatory in many regions including the European Union (EU). All companies trading must appoint a QPPV. Their role covers all aspects of the PV system to ensure regulatory standards are adhered to and any safety concerns are addressed promptly.
Qualifications and Skills of a Qualified Person for Pharmacovigilance (QPPV)
A QPPV needs a blend of scientific, regulatory, and managerial skills to perform their duties effectively. Below are some of the qualifications and skills that are typically required for this role:
1. Educational Background
- Bachelor’s Degree in Pharmacy, with additional certificate/diploma/fellowship or postgraduate training in good pharmacovigilance practices (GVP) from institutions recognized by the Pharmacy and Poisons Board.
- The qualified person responsible for Pharmacovigilance shall in addition receive a mandatory refresher GVP training facilitated by the MAH in accredited/recognized institutions by the Board.
- The refresher training shall be carried out at least once in two years and evidence of the same submitted to the Board upon request or during audits/inspections.
2. Experience and Knowledge
- Know applicable Kenyan safety monitoring legislation and guidelines and international standards for good pharmacovigilance practices.
- Demonstrate GVP knowledge in the implementation of activities stipulated in the MAHs pharmacovigilance system master file.
- The QPPV shall have a current letter of designation from the Pharmacy and Poisons Board as the QPPV pharmacist and must produce the same at the time of pharmacovigilance inspections.
- The QPPV shall be qualified by pertinent training or experience relevant to their assigned responsibilities.
- Familiarity with regulatory frameworks, especially the EU Good Pharmacovigilance Practice (GVP) guidelines, and regional pharmacovigilance requirements.
- The ability to interpret safety data, identify trends, and make informed decisions.
- A QPPV must liaise effectively with various departments, regulatory bodies, and healthcare professionals, which requires strong communication and leadership abilities.
Challenges and Future Perspectives Faced by a Qualified Person for Pharmacovigilance (QPPV)
1. Overseeing the Pharmacovigilance System
The QPPV is responsible for establishing and maintaining the pharmacovigilance system for their company or client. They ensure that all processes align with regulatory requirements, and they coordinate with other departments to manage risk and ensure that safety data is reported accurately and on time.
2. Signal Detection and Risk Management
One of the most crucial tasks of a QPPV is signal detection – identifying new safety information or trends related to adverse events. They assess risks associated with the medicinal products and implement measures to mitigate those risks through various tools like risk management plans (RMPs)
3. Compliance and Regulatory Reporting
QPPVs ensure compliance with regional and international pharmacovigilance regulations. They are responsible for submitting safety reports, such as Periodic Safety Update Reports (PSURs) and Individual Case Safety Reports (ICSRs), to regulatory authorities within strict deadlines. Failure to comply can lead to fines, penalties, or even product recalls, which can damage a company’s reputation.
4. Providing Pharmacovigilance Regulatory Intelligence
The QPPV monitors updates to pharmacovigilance regulations, guidelines, and legislation at the national, regional, and global levels. Analyzing how new or revised regulations impact the company’s pharmacovigilance system.
5. Conducting Pharmacovigilance Audits and Inspections
QPPVs play a pivotal role in preparing for and managing pharmacovigilance audits and inspections. They ensure that the pharmacovigilance system is inspection-ready and that documentation, processes, and data meet regulatory standards. These audits help identify gaps, risks, and areas for improvement.
6. Literature Screening/Monitoring
Literature screening involves a thorough examination of scientific literature, journals, and databases to identify relevant information about adverse drug reactions, safety concerns, and emerging trends. This proactive approach allows us to anticipate potential risks and take timely preventive measures.
7. Case Management
QPPV is responsible for collecting, entering, writing narratives, conducting medical assessments, and expediting the reporting of individual case safety reports (ICSR). Case management plays a vital role in guaranteeing the safety and effectiveness of pharmaceutical products.
Why is the Qualified Person for Pharmacovigilance (QPPV) Essential?
1. Patient Safety
Ensures that pharmaceutical products remain safe and effective for patients. Plays a critical role in preventing adverse drug reactions (ADRs) from going unnoticed, minimizing potential harm to the public.
2. Risk Management
Engages in risk assessment and signal detection to identify and address potential safety concerns.
3. Regulatory Reporting
Ensures compliance with regulatory requirements by managing timely and accurate pharmacovigilance reporting.
4. Public Trust
Maintains and enhances public trust in pharmaceutical companies by demonstrating a commitment to safety and regulatory compliance.
5. Data Transparency and Reliability
This meets the increasing demand for transparent and reliable safety data from patients and healthcare providers.
6. Central Role in Pharmacovigilance
Acts as the linchpin of a company’s pharmacovigilance activities, highlighting their pivotal role in the system.
Challenges and Future Perspectives Faced by a Qualified Person for Pharmacovigilance (QPPV)
The role of the QPPV continues to evolve with advancements in technology and data analytics. QPPVs are now leveraging artificial intelligence (AI) and machine learning to enhance signal detection and automate routine tasks, making pharmacovigilance systems more efficient and responsive. However, challenges remain;
1. Technological Adaptation
Keeping pace with advancements in Artificial Intelligence (AI) and Machine Learning while ensuring the tools are used effectively and appropriately.
2. Rapidly Changing Regulations
Staying updated with evolving regulations across different regions and adapting pharmacovigilance systems to comply promptly.
3. Data Privacy Concerns
Managing patient data securely while navigating complex global data privacy laws such as GDPR and other local regulations.
4. Global Landscape Complexity
Addressing diverse and sometimes conflicting regulatory requirements in a multinational context.
5. Balancing Innovation and Compliance
Integrating innovative technologies without compromising regulatory compliance or patient safety.
6. Skillset Requirements
Acquiring and maintaining expertise in emerging technologies, data analytics, and regulatory intelligence.
Conclusion
The Qualified Person for Pharmacovigilance is vital in safeguarding patient safety, ensuring regulatory compliance, and upholding public health. Their role in managing risks and maintaining robust systems enhances the quality of life for patients worldwide.
Jeyflex Consultants offers expert QPPV services tailored to meet the organization’s needs. With a commitment to excellence in pharmacovigilance, we provide comprehensive support to ensure compliance, mitigate risks, and uphold patient safety. Contact us today to strengthen your pharmacovigilance systems and build trust with your stakeholders.
Article by Marraret Ouma