If you work in pharmaceuticals or healthcare, you’ve likely heard the term’ Local Literature Monitoring‘ (LLM). It may sound technical, but at its core, it’s about keeping patients safe by making sure no important safety information slips through the cracks.
Think about it: not all research or case reports make it into big international journals like PubMed. Sometimes, the first clue about a side effect shows up in a local medical journal, a university bulletin, or even conference proceedings. That’s where local literature monitoring comes in.
So, what exactly is Literature Monitoring in Pharmacovigilance?
In simple terms, literature monitoring in Pharmacovigilance means regularly scanning scientific papers, articles, and case studies to check if there’s any new information about a medicine’s safety.
For pharmacovigilance, this isn’t just nice to have; it’s a requirement. Companies need to:
- Spot potential side effects (adverse drug reactions).
- Pick up on early safety signals.
- Report these findings to regulators within strict timelines.
It’s a structured, routine process that helps ensure patients aren’t put at unnecessary risk.
What Counts as Local Literature in Pharmacovigilance?
Local literature in pharmacovigilance is exactly what it sounds like: region-specific publications. These might not appear in international databases, but they can hold critical information. Some examples include:
- Medical journals published in a particular country.
- Articles in the local language that don’t get indexed globally.
- Research from local universities or hospitals.
- Abstracts from regional scientific meetings.
Imagine a rare side effect of a widely used drug being reported in a Kenyan or Indian medical journal. If no one is watching that source, the information could be missed globally, and patients would be the ones at risk.
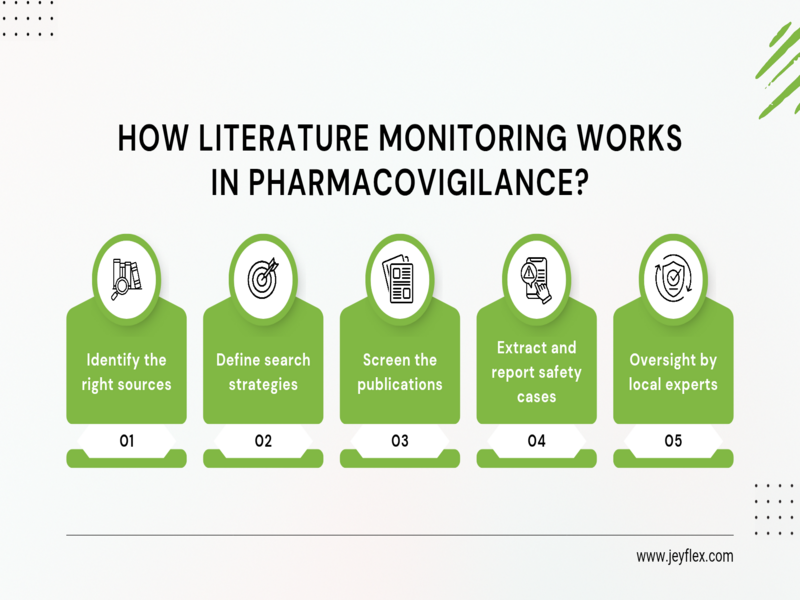
How Does Literature Monitoring Work in Pharmacovigilance?
Literature monitoring may sound complex, but in practice, it follows a structured and repeatable process designed to catch important safety information as early as possible. Here’s how it usually works:
Identifying the right sources
The first step is deciding where to look. This includes international databases, local medical journals, university publications, hospital newsletters, and even regional conference abstracts. Each company develops a list of “must-monitor” sources that are most relevant to their medicines and therapeutic areas.
Defining search strategies
Once the sources are chosen, pharmacovigilance teams create a set of search strategies. This usually means combining drug names, active ingredients, brand names, and medical terms related to adverse reactions. For example, if a company markets a pain reliever, searches might include both its chemical name and the brand name, plus terms like “liver toxicity” or “allergic reaction.”
Screening the publications
The next step is to carefully screen each article, case report, or study. Not everything will be relevant; some might just mention a drug in passing, so the team looks for content that provides meaningful safety information. This step requires both medical knowledge and attention to detail, since even a single case study could signal an important risk.
Extracting and reporting safety cases
If a publication does contain information about an adverse drug reaction, it is documented and assessed. Valid cases are converted into Individual Case Safety Reports (ICSRs), which must then be submitted to health authorities within strict timelines (often 15 days for serious cases).
Oversight by local experts
To make sure the process runs smoothly and meets regulatory expectations, companies may appoint a Local Qualified Person for Pharmacovigilance (L-QPPV). This person is responsible for overseeing the local literature review, ensuring compliance, and acting as the key contact for regulators in that region.
Global Literature Monitoring: The Bigger Picture
Beyond local sources, regulators also expect companies to monitor global literature. This means keeping track of internationally recognized databases, scientific journals, and research publications.
Agencies like the European Medicines Agency (EMA) and the FDA specifically require companies to capture:
- Individual case safety reports (ICSRs).
- Emerging safety signals.
- Publications with broader scientific or clinical relevance.
For example, imagine a cardiology study published in a European journal that mentions a patient developing heart rhythm issues after using a certain drug. Even if that drug is marketed in Africa or Asia, the company responsible for it must capture and report this information — because risks do not respect borders.
In short, local literature monitoring protects against what’s happening in your backyard, while global monitoring ensures you don’t miss what’s happening worldwide. Both are essential pillars of pharmacovigilance.
Challenges in Literature Monitoring
Despite its importance, literature monitoring in pharmacovigilance isn’t without hurdles:
- Language barriers: Local journals may be published in regional languages.
- High publication volumes: Thousands of papers are published daily, making it easy to miss something.
- Relevance filtering: Many articles mention drugs casually without providing meaningful safety data.
This is why many companies are turning to technology, automation, and AI tools to make the process faster, more reliable, and less resource-intensive.
Why Literature Monitoring Matters Beyond Compliance
It’s easy to view literature monitoring as just a regulatory checkbox, but it’s much more than that. It’s about protecting patients, supporting healthcare providers, and maintaining trust.
When companies identify signals early, they can:
- Update product labels.
- Inform doctors and patients faster.
- Prevent harm before it spreads.
Ultimately, literature monitoring in pharmacovigilance helps ensure that medicines do more good than harm.
Take Away
Whether it’s a single case study in a local hospital bulletin or a multi-centre trial in an international journal, literature monitoring in pharmacovigilance ensures no safety signal is overlooked. By combining local vigilance with global awareness, pharmaceutical companies can protect patients and stay compliant with strict regulatory requirements.
At Jeyflex Consultants, we help pharmaceutical companies set up and manage both local and global literature monitoring systems. From defining search strategies to ensuring timely ICSR submissions, we make sure no critical safety signal slips through.
If you’d like to strengthen your pharmacovigilance systems, get in touch with our team today.
Article by Marraret Ouma